Our Take: Humana launches new oncology payment model

Humanaannouncedthat it has launched a national value-based care oncology program for its commercial and Medicare Advantage members.
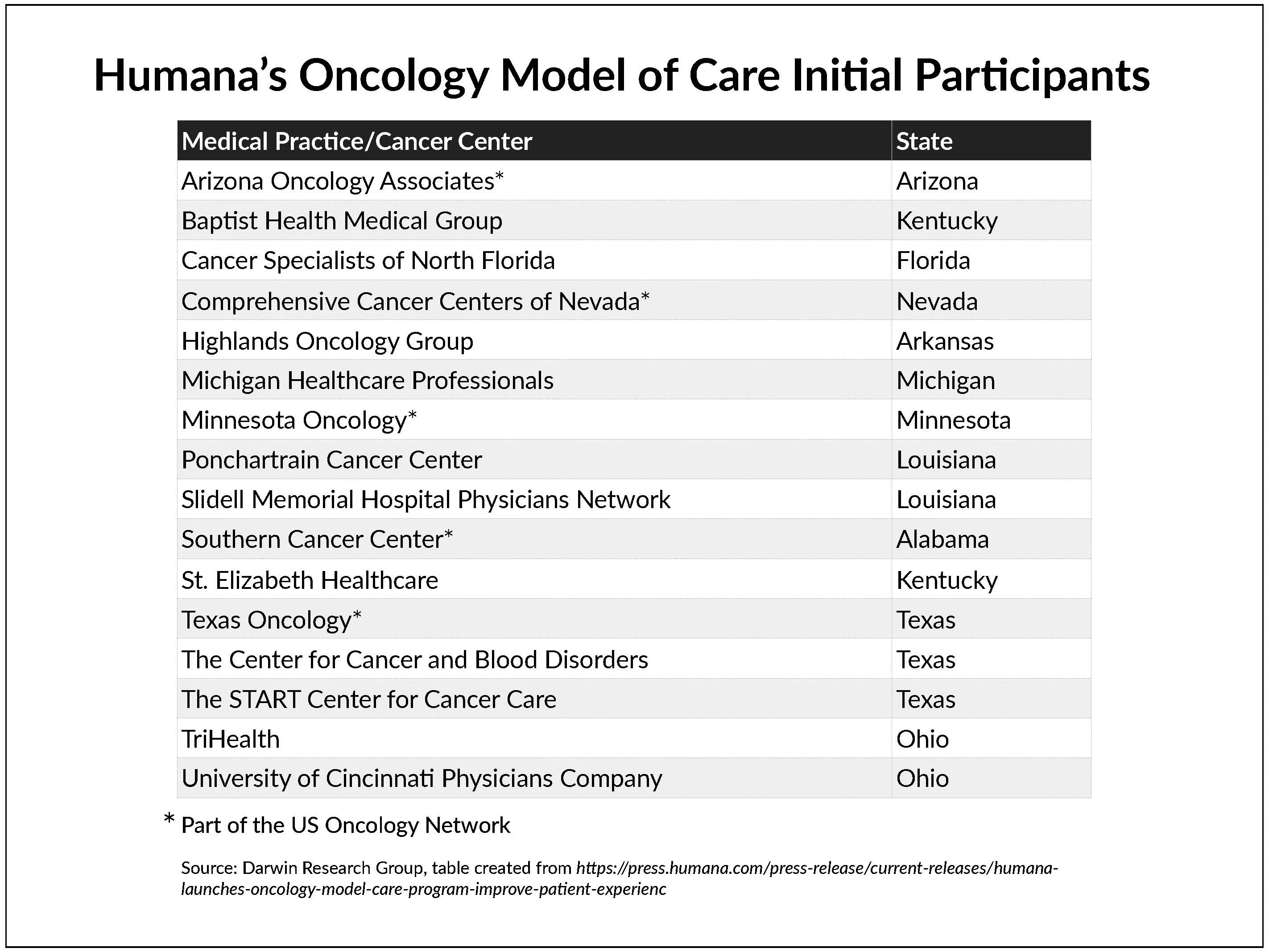
What else you need to know
Chronic conditions cost the U.S. economy over $4.7 trillion annually, and that number is likely to double in the next three decades as baby boomers continue to age, according to a report by Fitch Solutions. Direct costs (e.g., hospital care, physician visits, drugs, medical devices and home care) for the leading chronic conditions — which include Alzheimer’s disease, arthritis, cancer, cardiovascular disease, diabetes and obesity — are more than $1 trillion per year. Indirect costs (e.g., loss of income, lower productivity, early retirement and premature mortality) are about $3.7 trillion.
The Senate Finance Committee has requested an investigation of price spreading practices by pharmacy benefit managers (PBMs). The committee’s top members, Sens. Chuck Grassley, R-Iowa, and Ron Wyden, D-Ore., sent a letter to the Office of the Inspector General about “concerning practices by [PBMs] in state Medicaid programs.” Price spreading is when a PBM charges a payer more for a drug than the PBM paid a pharmacy in reimbursement. In the letter, the senators pointed to results from an audit of Ohio’s Medicaid program as an example of their concern; the audit found that PBMs made more than $224 million in a year’s time via price spreading.
Sutter Health and the University of Pittsburgh Medical Center (UPMC) are partnering with DNAnexus to develop personalized treatments for multiple sclerosis (MS). Researchers at Sutter Health’s Center for Precision Medicine will collect clinical data from more than 3,000 patients with MS, and the UPMC Genome Center will generate genomic data from participating patients’ samples. The data sets will be processed and linked on DNAnexus’ Apollo platform. The first phase of the study is set to begin in May. Separately, a recently published study of 241 patients with an established diagnosis of MS found that 18% of the patients did not actually have MS but instead had some other condition such as migraine.
CMS is encouraging the use of generic drugs in Obamacare health plans by including a provision in a new rule that will allow insurers to dissuade plan members from using manufacturers’ coupons to lower their out-of-pocket costs for certain brand-name drugs when a generic alternative is available. To steer plan members toward the generic versions, starting in the 2020 plan year, insurers will be allowed to advise members that their copays will not count toward their annual cost-sharing limit if the member chooses the brand-name drug and uses a manufacturer’s coupon to lower the cost.
The FDA approved the first targeted therapy for metastatic bladder cancer,Janssen Pharmaceuticals’ Balversa (erdafitinib). The drug, which received accelerated approval, is indicated for use in adults with locally advanced or metastatic bladder cancer that has an FGFR3 or FGFR2 genetic alteration and has progressed despite chemotherapy. A companion diagnostic device was approved in conjunction with the drug’s approval.What we're reading
Value-Based Care in America: State-by-State. Change Healthcare, 2019
The Uncertain Effect of Financial Incentives to Improve Health Behaviors. JAMA 3.25.19
New Evidence on Stemming Low-Value Prescribing. NEJM Catalyst, 4.10.19
Darwin's Our Take: Feature: A rare FDA refusal, CDC grant cuts, and proposed ACA exchange changes

Darwin's Our Take: Lilly’s, Novo Nordisk’s share prices roller-coaster following earnings reports

Darwin's Our Take: Botox, Trulicity, Xolair among drugs selected for next round of Medicare price negotiations

Subscribe to Our Take
Sign up for Our Take Newsletter: highly curated, expert weekly strategic insights for health care executives.

